Tenofovir Gel for the Prevention of HSV-2 Infection
Data from the CAPRISA 004 trial showing that pericoital application of tenofovir gel, an antiviral microbicide, reduced Herpes Simplex type 2 (HSV-2) acquisition by 51% in women was recently published in the New England Journal of Medicine.
Globally, Herpes simplex virus type-2 (HSV-2) is among the most common sexually transmitted infections and is the leading cause of genital ulcers. Available global estimates indicate that approximately 536 million (16.5%) sexually active adults between the ages of 15 and 49 years were infected with HSV-2 in 2003.
HSV-2 is a lifelong infection that causes recurrent painful genital ulceration and potentially fatal herpes infections in newborns. It is also associated with a 3.4-fold increased risk of HIV acquisition in women, after adjustment for sexual behaviour. Interventions to prevent HSV-2 infection, including condoms, circumcision, and antiviral treatment, have demonstrated protection levels ranging from 6% to 48%.
The effectiveness of pericoital tenofovir gel in preventing HSV-2 acquisition was assessed in a subgroup of 422 HSV-2 negative women enrolled in the CAPRISA 004 study.
Pericoital tenofovir gel reduced HSV-2 incidence (as measured by ELISA) by 51% overall (95% CI: 23-70%, P = 0.003). Confirmatory testing using Western blot testing produced similar results ie 55% effectiveness (95% CI: 18-77%; P = 0.005).
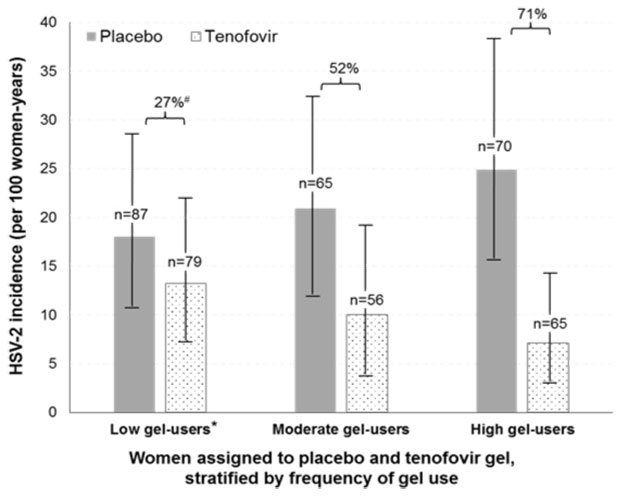
Risk of HSV-2 acquisition was reduced by 71% in women with high gel-use (defined as returning >6 applicators per month) compared to 27% reduction in the women with low gel-use (<4 applicators/month) (Figure)
Since there is no effective vaccine or cure for HSV-2, pericoital tenofovir gel has the potential to increase the range of options for HSV-2 prevention programs, which at present promote condoms and circumcision.
Effective prevention strategies for HSV-2 infection are needed to achieve the goals of the World Health Organization global strategy for the prevention and control of sexually transmitted infections.
For further reading see:
Abdool Karim SS, et al New Engl J Med 2015; 373: 530-9. http://www.nejm.org/doi/full/10.1056/NEJMoa1410649
