Mucosal antibody responses in women using TFV gel - implications for HIV vaccines
Research by the Mucosal Immunology research team was recently published in the journal Mucosal Immunology, which is is part of the Nature group of journals. The study showcases the genital tract and blood HIV-specific antibody responses in women with breakthrough HIV-infections from the CAPRISA 1% tenofovir microbicide gel trial.
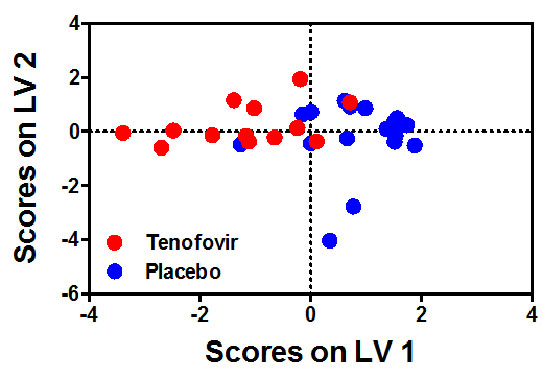
This is the first study to show in detail and in parallel, the kinetics and evolution of antibody responses the blood and genital tract from the primary into the chronic infection state. In this study, Dr Archary et al., showed that women that were exposed to the tenofovir gel had higher antibody responses rates to Env gp120, p66 and p24 in both the blood and genital tract. Additionally, women in the tenofovir gel arm had increased magnitudes of p66 IgG and IgA antibodies in both compartments compared to the women from the placebo arm (Figures A and B). Taken together, these data suggest that humoral immune responses are increased in blood and genital tract of individuals who acquire HIV infection in the presence of tenofovir gel and that prior tenofovir gel usage did not ablate or dampen subsequent humoral immunity in either compartments.
This study informs on strategies where vaccines and PrEP will be used in combination to prevent HIV. This study was a collaboration between CAPRISA, and the Duke Human Vaccine Institute. Dr Archary was given an opportunity to train at Duke University under the mentorship of Dr Tomaras and Dr Yates on the customized binding antibody multiplex assay (BAMA), a technique that is widely used in vaccine research at the HVTN to screen for multiple vaccine immune responses. Dr Archary has subsequently transferred the technology to CAPRISA’s Mucosal Immunology Laboratory, and is currently using this technique a wide range of other studies.
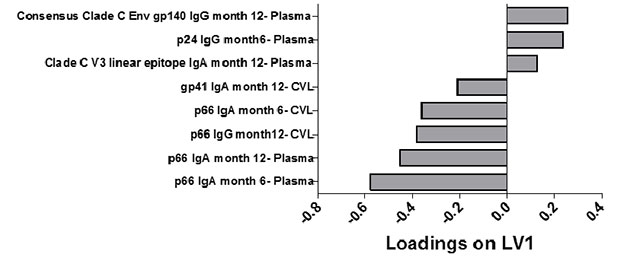
Figure A and B: Six and 12 month antibody signatures associated with participants in the TFV and placebo arms. LASSO identified a signature of 8 antibody specificities that separated TFV (red- n=13) and placebo (blue-n=18) arms in a PLSDA model with 83% calibration accuracy and 77% cross-validation accuracy (scores plot; A). The loadings plot depicts weighted loadings of individual antibody specificities within the distinguishing signature (B). Five of these specificities were positively associated with TFV (negatively loaded on LV1; plasma p66 IgA at months 6 and 12, plasma p66 IgG at month 12, CVL p66 IgA at month 6, and CVL gp41 IgA at month 12) and three were negatively associated with TFV (positively loaded on LV1; plasma Clade C V3 linear epitope IgA at month 12, p24 IgG at month 6 and Consensus Clade C Env gp140 IgG at month 12.
